What are Septins?
Together with the microtubule, microfilament and intermediate filament networks, the septins constitute the fourth component of the eukaryotic cytoskeleton (Reviewed in (Mostowy and Cossart, 2012)). They were initially identified in yeast as crucial mediators of cytoplasmic division during budding or septum formation (Reviewed in (Saarikangas and Barral, 2011)). Septin genes have been identified in almost all organisms except higher plants and certain algae and were shown to have an evolutionarily conserved role in cytokinesis (Figure 1A & B). The 13 members of septin GTPase family (Figure 1C)expressed in mouse/human are classified into 4 groups based on their sequence homology, which include SEPT3 group (SEPT3, 9 & 12), SEPT2 group (SEPT1, 2, 4 & 5), SEPT6 group (SEPT6, 8, 10, 11 & 14) and SEPT7 group (SEPT7). Septins form non-polar heteropolymers which can take complex and unique higher order structures like rings and cages (Figure 1D- 1E). The most well characterized basic repeating units of the septin cytoskeleton consists of a hexamer [SEPT7:6:2:2:6:7] or octamer [SEPT9:7:6:2:2:6:7:9], wherein the individual subunits can be replaced by members of the same group (Figure 1D). Recent studies have revised the octameric repeating unit to be in the order "SEPT2–SEPT6–SEPT7–SEPT7–SEPT6–SEPT2" (Mendonça et al., 2019). SEPT7 forms the unique and irreplaceable component of the septin cytoskeleton, depletion of which leads to complete absence of the septin cytoskeleton in all cell types analyzed so far. Studies have confirmed the role of septin isoforms in different stages of mammalian cell division including chromosome segregation and cytokinesis (Kinoshita et al., 1997), Bacterial pathohenesis (Mostowy et al., 2010), calcium signaling (Sharma et al., 2013) etc [More details at the Frontiers Research Topic: Emerging Functions of Septins].

Figure 1. Septins: Fourth component of cytoskeleton. A. Septin double-ring in budding yeast, marking the bud-neck. B. SEPT7 localization during fibroblast cytokinesis. C. Domain organisation of septins depicting proline rich domain (PRD), Polybasic region (PBR), GTP-binding domain, septin unique element (SUE) and coiled-coil region (CC). D. Hetero-oligomeric complexes (hexamer / octamer) of septins formed by alternate N-C and G-G interfaces. E. Higher order septin assemblies. (Modified from Menon, 2018 (Menon, 2018))
Major conclusions from own work
Our work in the past couple of years could establish the concept of ``cell-type specificity`` of septin functions and have generated interest on septins as targets for anti-tumor therapy (Menon and Gaestel, 2015; Menon et al., 2014) (Figure 2). Major conclusions from my past research in the septin field are summarized below.
Septins are critical for embryogenesis and fibroblast cytokinesis: To characterize the in vivo functions of mammalian SEPT7, a Sept7 conditional allele was generated by targeting the exon 4 of the mouse Sept7 gene, which encodes critical residues of the GTPase domain. Sept7 knockout (KO) embryos displayed early embryonic lethality between days E7.0 and E10.5 and septin-depleted fibroblasts underwent obligate multinucleation. Further studies could confirm abscission defects in Sept7-KO fibroblasts which could be attributed to altered microtubule stability (Menon et al., 2014). Septin cytoskeleton is dispensable for hematopoiesis: By use of multiple gene targeting approaches, it was shown that general hematopoiesis is independent of septins (Menon et al., 2014 and unpublished data). This important finding makes septin-dependent cytokinesis a plausible target for therapeutic intervention against solid tumors without undesired side-effects on hematopoiesis (Menon and Gaestel, 2015; Menon et al., 2014) (Figure 2B). Further studies using a tumor model of septin-deletion, we could establish the proof of concept finding that septin targeting can suppress lung tumorigenesis (Menon et al., 2021).
GTPase domain dependent dimerization of SEPT7 is dispensable for fibroblast cytokinesis: Septins are GTPases and the GTPase (G) domains of septins are an integral part of the polymerization interface for the hetero-polymer assembly (Reviewed In Abbey et al., 2019). Catalytic domains are the best targetable nodes for small-molecule intervention, mainly due to their accessibility and possible substrate mimicry. This necessitates better understanding of septin polymerization. In vitro studies have established the role of G-domain dependent SEPT7: SEPT7 dimerization in the formation of septin filaments from the hexameric SEPT7/6/2/2/6/7 subunits. By use of a newly generated SEPT7-KO rescue model and a panel of SEPT7 mutants we could show the dispensability of SEPT7 G-domain interface in fibroblast cytokinesis and propose SEPT7-SEPT9 interface as a possible target for inhibiting the process (Abbey et al., 2016). We are currently investigating the potential pharmacologically targetable septin interfaces and physiologically relevant septin modifications (Sharma & Menon, 2023).
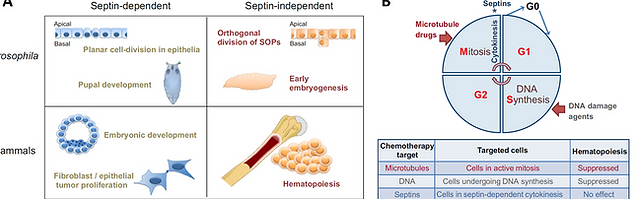
Figure 2. Cell-type specific roles of septins in cytokinesis and targetability of septin-dependent cytokinesis against cancer. A. The scheme summarizes the findings from Drosophila and mammalian systems indicating a cell-type specific role for septins in cell division. B. The commonly used anticancer drugs target the mitotic / DNA-synthesis phases of the cell cycle in actively dividing cells in the body, which includes hematopoietic cells in addition to cancer cells. Development of septin–dependent cytokinesis inhibitors will open a new therapeutic approach to specifically target solid tumor cells, thus leaving hematopoiesis unaffected. (Figures modified from: Menon & Gaestel, J Cell Science, 2015).
References / Further reading
Abbey, M., Hakim, C., Anand, R., Lafera, J., Schambach, A., Kispert, A., Taft, M.H., Kaever, V., Kotlyarov, A., Gaestel, M.*, & Menon, M.B.* (2016). GTPase domain driven dimerization of SEPT7 is dispensable for the critical role of septins in fibroblast cytokinesis. Sci Reports 6, 20007.
Abbey, M., Gaestel, M.* & Menon, M.B.* (2019). Septins – active GTPases or just GTP-binding proteins? Cytoskeleton. 76: 55– 62.
Kinoshita, M., Kumar, S., Mizoguchi, A., Ide, C., Kinoshita, A., Haraguchi, T., Hiraoka, Y., and Noda, M. (1997). Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 11, 1535-1547.
Menon, M.B.*, Sawada, A., Chaturvedi, A., Mishra, P., Schuster-Gossler, K., Galla, M., Schambach, A., Gossler, A., Forster, R., Heuser, M., et al. (2014). Genetic deletion of SEPT7 reveals a cell type-specific role of septins in microtubule destabilization for the completion of cytokinesis. PLoS Genet 10, e1004558.
Menon, M.B.*, and Gaestel, M. (2015). Sep(t)arate or not - how some cells take septin-independent routes through cytokinesis. Journal of cell science 128, 1877-1886.
Menon, M.B. (2018). Septin. In Encyclopedia of Signaling Molecules, S. Choi, ed. (Cham: Springer International Publishing), pp. 4875-4884.
Menon M.B.* & Gaestel M*, eds. (2017). Emerging Functions of Septins. Lausanne: Frontiers Media. DOI: 10.3389/978-2-88945-287-3.(eBook)
Menon M.B., Yakovleva T, Ronkina N, Suwandi A, Odak I, Dhamija S, Sandrock I, Hansmann F, Baumgärtner W, Förster R, Kotlyarov A, Gaestel M. Lyz2-Cre-Mediated Genetic Deletion of Septin7 Reveals a Role of Septins in Macrophage Cytokinesis and Kras-Driven Tumorigenesis. Front Cell Dev Biol. 2022;9:795798.
Mendonça DC, Macedo JN, Guimarães SL, Barroso da Silva FL, Cassago A, Garratt RC, Portugal RV, Araujo APU. A revised order of subunits in mammalian septin complexes. Cytoskeleton (Hoboken). 2019;76(9-10):457-466.
Mostowy, S., Bonazzi, M., Hamon, M.A., Tham, T.N., Mallet, A., Lelek, M., Gouin, E., Demangel, C., Brosch, R., Zimmer, C., et al. (2010). Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe 8, 433-444.
Mostowy, S., and Cossart, P. (2012). Septins: the fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 13, 183-194.
Saarikangas, J., and Barral, Y. (2011). The emerging functions of septins in metazoans. EMBO Rep 12, 1118-1126.
Sharma, S., Quintana, A., Findlay, G.M., Mettlen, M., Baust, B., Jain, M., Nilsson, R., Rao, A., and Hogan, P.G. (2013). An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca entry. Nature. 499:238-42.
Sharma K & Menon M.B.* Decoding post-translational modifications of mammalian septins. Cytoskeleton (Hoboken). 2023;80(7-8):169-181.
Special Issue on Septin Cytoskeleton: Cytoskeleton. 76 (1). January 2019
